Holaira Inc has begun treatments in the AIRFLOW-1 Clinical Study for patients with moderate to severe chronic obstructive pulmonary disease (COPD). The study is using a procedure called Targeted Lung Denervation (TLD).
TLD is a one-time bronchoscopic therapy performed with the Holaira System using the dNerva Dual Cooled RF Catheter. The catheter’s proprietary design delivers precisely targeted cooled radiofrequency energy to provide ablative therapy that denervates parasympathetic signals to the whole lung, the company reports.
Parasympathetic denervation results in smooth muscle relaxation throughout the bronchial tree, which can open obstructed airways to improve breathing, and has the potential to provide lasting whole lung improvement for COPD patients.
Using the Holaira Lung Denervation System, the first patient was treated at Otto Wagner Spital in Vienna, Austria.
“The first patient treated, a 72 year old male with COPD, tolerated the therapeutic treatment extremely well,” said Arschang Valipour, MD, PhD, FCCP, group leader at the Ludwig-Boltzmann Institute for COPD and Respiratory Epidemiology in the Department of Respiratory and Critical Care Medicine at Otto Wagner Spital. “TLD was safely delivered to both lungs in a single procedure with no procedural complications. As with our earlier feasibility work, we anticipate the patients in the AIRFLOW-1 study will experience a sustained breathing improvement. The prospect of providing a lasting clinical improvement while expanding treatment options for these patients would be a significant breakthrough for the pulmonary community.”
AIRFLOW-1 (NCT#02058459) is a sequential two-phase multicenter, randomized study to optimize dose selection and evaluate safety after treatment with the Holaira Lung Denervation System in patients with moderate to severe COPD. It is the first randomized clinical trial with a sham control for Targeted Lung Denervation. The Phase II, 125 patient study is taking place at 15 institutions in Austria, Belgium, France, Germany, The Netherlands and the United Kingdom.
“The initiation of this clinical study is an important milestone in our work to develop the first lung denervation system that addresses the unmet needs of patients who battle obstructive lung diseases on a daily basis,” said Dennis Wahr, MD, chief executive officer of Holaira. “The AIRFLOW-1 study will broaden our clinical experience among leading pulmonary facilities in the European Union, while demonstrating a potentially alternative treatment option for COPD sufferers that may have a lasting effect compared to current pharmacological treatment.”
Prior to initiation of the AIRFLOW-1 study, Holaira completed multiple preclinical studies and two multicenter human feasibility studies. One-year results of these studies were presented last month at the European Respiratory Society International Congress (ERS) in Munich. Results from these earlier 37-patient trials demonstrated safety and feasibility of the Holaira System for TLD in patients with moderate to severe COPD. Evidence of the potential clinical benefit of this novel technique was shown by durable patient improvements in lung function, exercise tolerance and quality of life.
*Targeted Lung Denervation (TLD) is currently under clinical investigation for chronic obstructive pulmonary disease and is not yet commercially available.

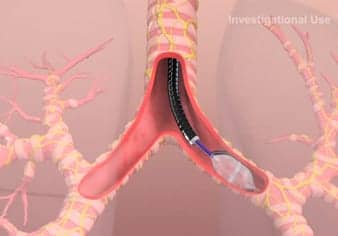









What is the size of the catheter? What is it made out of?