This article aims to define four cyanotic cardiac defects in the pediatric population along with a discussion of the effects of oxygen on both the unrepaired and repaired cardiac anomaly.
By Roy Ramirez, RCP, RRT-NPS, RPFT; Troy Taylor, RCP, CRT; Mindy Pera, RCP, BS, CRT; Maila Factora, RCP, MBA; and John Cleary, MD
In 1774, Joseph Priestley, an English amateur chemist, was credited with discovering oxygen by intensely heating mercuric oxide over liquid mercury in a closed vessel. Although Swedish apothecary Karl W. Scheele had independently discovered oxygen two years before, his delay in publication (1777), along with a lack of detailed experimentations on the nature of the gas, lost him total credit for the discovery.1
Therapeutic use of the gas for the first 150 years was sporadic at best, primarily attributed to inadequate delivery devices as well as a skeptical medical community. It was not until more than 100 years later that findings on the therapeutic value of oxygen were published and accepted by practitioners for use in medical intervention and treatment of their patients.
Inquiries regarding the dangers of gas inhalation were first brought up in 1870, and the response by the medical community at that time was that the gas could be used without any possible risk of harm to the patient.1 We know today that this is not true. Like any other drug, an inappropriate dose and use combined with a failure to adequately monitor the patient can result in a poor outcome and, at times, can be even extremely detrimental to the patient.
This article aims to define four cyanotic cardiac defects in the pediatric population along with a discussion of the effects of oxygen on both the unrepaired and repaired cardiac anomaly. Cyanotic congenital heart disease (CHD) patients grow and thrive at oxygen saturations that would be concerning in other pediatric patients. Prior to birth, however, the fetus has developed in an environment with PaO2 of about 30. Oxygen delivery to the tissues is dependent on cardiac output, hemoglobin level, and oxygen content of the blood—not simply the saturation.
In the CHD patient, if arterial and venous blood are mixing completely or if there is shunting of blood from the venous system to the arterial system (R to L shunt), a reduced saturation is anticipated. Therefore, an understanding of the appropriate saturation target for each patient is imperative.
The hypoplastic left heart syndrome (HLHS) is an example of complete mixing with a ductal dependent cardiac output. Blood returning from the healthy lungs (SaO2 >95%) mixes with venous blood (SvO2) (typical SvO2 about 65%). If equal volumes of blood are distributed to the lungs and to the body (qP/qS=1 or normal), a saturation of about 80 would be expected. If increased blood goes to the lungs rather than to the body (ie, two or three times as much well-saturated blood mixing with venous blood), the arterial saturation will rise, but the cost may be increased work for the heart and/or a reduced cardiac output. Interventions that lower the pulmonary vascular resistance (PVR) and increase pulmonary blood flow will worsen this problem, and oxygen is a potent dilator of the pulmonary circulation.
Cyanotic Cardiac Defects
Hypoplastic Left Heart Syndrome. One of the most challenging congenital heart defects to manage is HLHS. The left side of the heart, including the aorta, aortic valve, left ventricle, and mitral valve, which receives oxygen-rich blood, is underdeveloped, leaving the left side of the heart unable to adequately support systemic circulation. In order for blood to return to the lungs, it must pass through the atrial septal defect (ASD). The right ventricle takes over pumping blood to the lungs (via pulmonary artery) and out the body patent ductus arteriosus (PDA). If HLHS is not diagnosed early and oxygen levels are not monitored to keep the ductus open, the patient will most often die within the first days of life.7

During diagnosis of a patient suspected of HLHS, the practitioner will observe rapid respiratory rates and cyanosis with saturations of 75% to 85% in newborns. Changes will occur as the PDA starts to close. Systemic perfusion will decrease rapidly, the patient will become lethargic, and pulses will diminish. Prostaglandin E1 (PGE1), along with sub-atmospheric concentrations of oxygen, is used to keep the PDA open. As with other single ventricle defects, using high levels of oxygen could also lead to excessive pulmonary blood flow and congestive heart failure (CHF), which are already precautionary factors in HLHS.
Three stages of treatment are required for repair of a patient with HLHS. The first stage—called a Norwood procedure—completed during the first days of life, creates systemic blood flow by anastomosing the proximal main pulmonary artery to the hypoplastic ascending aorta. At 6 to 8 months of life, a bidirectional Glenn procedure is performed. The final stage of the repair is the Fontan procedure. After the first two procedures, the ductus can safety close; subambient air and PGE1 can be discontinued. The Fontan procedure improves system oxygenation by decreasing mixing of oxygenated and deoxygenated blood, resulting in an increase in saturation to 85% and 90% with a fenestration and 100% without fenestration.
Double Outlet Ventricle. Double outlet right ventricle (DORV) and double outlet left ventricle (DOLV) are congenital heart defects in which both the pulmonary artery and aorta originate from the right or the left ventricle. Both DORV and DOLV have a ventricular septal defect (VSD) of the other ventricle in common. DORV or DOLV accounts for only 0.05% to 1.5% of patients with congenital heart disease.
Pulmonary stenosis occurs in 50% of patients with this anomaly, resulting in a patient with a blue discoloration (cyanosis). Pulmonary blood flow is dependent on the degree of stenosis: The greater the restriction of the pulmonary artery, the less blood will flow to the lungs for oxygenation.2 Some degree of pulmonary stenosis protects the pulmonary capillary bed from being exposed to high pressures and flows. Oxygenation is dependent on the ratio of pulmonary to systemic blood flow, and in the absence of pulmonary stenosis, the pulmonary capillary bed can be influenced by administration of oxygen. High levels of oxygen can decrease PVR, resulting in the potential for pulmonary edema.
When presented with a cyanotic patient with an unknown diagnosis, clinicians must take care when administering oxygen. Keeping in mind that oxygen is a pulmonary vasodilator, administration should be titrated to maintain a patient saturation between 75% and 85%. Using an oxygen blender is highly advised, since both FiO2 and flow can be controlled.
Caution must be taken to not allow a saturation of greater than 85% in patients with DORV on oxygen therapy, since higher saturations will potentiate pulmonary capillary vasodilatation, leading to pulmonary overcirculation. This can eventually lead to right heart failure.
Prior to surgical repair, intervention may include administering oxygen through a nasal cannula to maintain the targeted range. Subambient air, a concentration of 10% oxygen and 90% nitrogen, may be required for those patients with saturations greater than 85% in room air.
The most common surgical intervention is an interventricular tunnel created from the left ventricle to the aorta. This procedure directs blood flow from the left ventricle to the aorta. In the postoperative stage, FiO2% concentration should be adjusted to maintain targeted saturations between 75% and 85% to avoid the risk of increasing the pulmonary to systemic flow ratio.2
Tricuspid Atresia. Tricuspid atresia (TA) is one of the most common cyanotic heart defects in the pediatric population. TA accounts for 1% to 3% of all congenital heart defects, making it the third most common form of cyanotic heart disease.3 The concern with tricuspid atresia is the improper development of the tricuspid valve, which prevents deoxygenated blood from the right atrium entering the right ventricle leading to the lungs.
Pulmonary blood flow in a patient with TA is dependent on a PDA. Normally, the PDA closes with the first breaths of life, therefore patients exhibiting such symptoms as cyanosis, rapid and labored breathing, tachycardia, and cool clammy skin should create suspicion of a possible cardiac defect, such as TA. In such patients, PGE1 should be administered early to maintain PDA patency. PGE1 will also help prevent pulmonary edema and systemic acidosis. Apnea is a side effect of PGE1, so practitioners can expect to place these patients on mechanical ventilatory support. High levels of oxygen may promote ductal closure so care must be given when administering it.
Oxygenated blood via the PDA mixes with deoxygenated blood from a left to right shunt through an ASD. The oxygen saturation for this patient will be approximately 75%. As with the double outlet defect, the practitioner should use an oxygen blender to maintain targeted saturation between 75% to 85% to avoid the risk of increased pulmonary blood flow and CHF.
Treatment requires three surgical procedures. The first surgery temporarily provides pulmonary blood flow through a Blalock-Taussig (BT) shunt. The patient will still experience low saturations due to blood mixing. Oxygen should be used to maintain the above targeted saturation. At 4 to 12 months, a Glenn shunt replaces the BT shunt. The procedure connects the superior vena cava to the right pulmonary artery. The inferior vena cava (IVC) remains connected to the heart. Mixing will still occur after this procedure, so saturations will continue to be lower. The practitioner should continue to use a minimal oxygen concentration strategy. The Glenn shunt creates the connections for the Fontan procedure (or Fontan completion). This final procedure, which redirects blood from the IVC to the lungs as well, improves the oxygenation of the blood.4 Saturations for this patient should be greater than 90%.
Heterotaxy. There are two groupings of heterotaxy: polysplenia (multiple spleens) and asplenia (no spleen). Polysplenia or asplenia is present in 3% of patients with structural heart disease.5 This patient population exhibits multiple heart defects; the most common are ASD and single ventricle.
Eighty percent of patients with polysplenia will have an ASD, while only 10% will have a single ventricle cardiac defect. Corrections of the heart defects are usually elective procedures in the first 5 years of life. The asplenia group has a 90% incidence of ASD and 50% incidence of single ventricle.6
The most common problem with heterotaxy is not enough blood flow to the pulmonary circulation. The treatment is a surgical intervention to create a systemic to pulmonary conduit. Administration of oxygen will decrease PVR and aid in pulmonary blood flow. Oxygen should be delivered to maintain saturations between 75% and 85% via blender to control FiO2. The use of subambient air might be required for saturation greater than 90% to inhibit pulmonary overcirculation by increasing PVR.
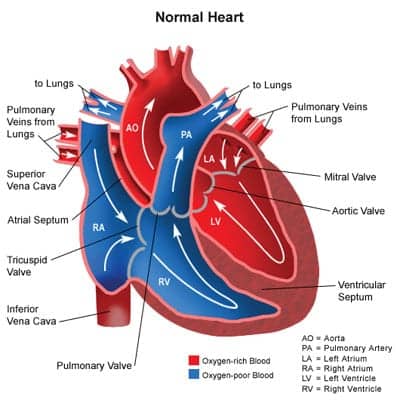
Conclusion
When dealing with the cardiac compromised pediatric patient, clinicians must understand that oxygen therapy can put a patient at risk. High concentrations of oxygen for cyanotic defects such as double outlet ventricle, heterotaxy, tricuspid atresia, and hypoplastic left heart syndrome can cause an increase in pulmonary blood flow leading to pulmonary overcirculation and right heart failure. Targeted saturations for this type of patient are between 75% and 85%. The use of sub-ambient air may be required for those patients with a higher saturation to achieve an FiO2 less than 21% to keep within the targeted range.
Defects that are ductal dependent will require the use of PGE1 to maintain patency. A side effect from this medication is apnea, requiring in most cases elective intubation. Stable patients on PGE1 are still at risk for increased blood flow to the lungs, so oxygen therapy should be administered conservatively.
RT
Roy Ramirez, RCP, RRT-NPS, RPFT, is clinical educator, respiratory services; Troy Taylor, RCP, CRT, is respiratory therapist supervisor, med/surg ECLS coordinator; Mindy Pera, RCP, BS, CRT, is clinical educator, respiratory services; Maila Factora, RCP, MBA, is administrative director, respiratory services; and John Cleary, MD, is associate director, cardiovascular intensive care and ECLS director, neonatology, Children’s Hospital of Orange County, Orange, Calif.
References
- Martin L. 1998. Oxygen therapy: the first 150 years. Available at: www.lakesidepress.com/pulmonary/papers/ox-hist/intro.html. Accessed January 31, 2008.
- Silka MJ. Double-outlet ventricles. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore: Williams & Wilkins; 1997:1505-23.
- Westmoreland D. Critical congenital cardiac defects in the newborn. Perinat Neonatal Nurs. 1999;12:67-87.
- Green A, Pye S, Yetman AT. The physiologic basis for and nursing considerations in the use of subatmospheric concentrations of oxygen in HLHS. Adv Neonatal Care. 2002;2:177-86.
- Rowe RD, Freedom RM, Mehrize A, Bloom KR. The Neonate with Congential Heart Disease. Philadelphia: WB Saunders; 1981:484.
- Van Mierop LHS, Gessner IH, Schiebber GL. Asplenia and Polysplenia Syndromes. Birth Defects. Baltimore: Williams & Wilkins; 1995:74-82.
- Heart Center Encyclopedia. 2006. Hypoplastic left heart syndrome. www.cincinnatichildrens.org/health/heart-encyclopedia/anomalies/hlhs.htm. Accessed February 1, 2008.