Quality spirometric monitoring includes proper training of technologists, continued competence assessment, and review of test results.
Over the past year, several guidelines and recommendations have been published that emphasize the need for quality monitoring of spirometry to achieve valid test results. In a 1999 study1 from New Zealand, Eaton and colleagues examined the quality of spirometry performed in primary care offices. This was the first study to formally address the quality of spirometry in the physician’s office. In an analysis of 1,012 spirometry tests from 30 primary care practices, only 3.4% of patients in practices where the staff perform tests with no training and 13.5% of patients in practices with staff that received minimal training had three acceptable maneuvers with a reproducible test. The primary reason for failure was lack of a plateau on the volume-time curve.
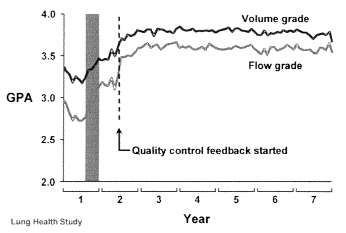
An abstract presented at the 1999 American Association for Respiratory Care International Congress reviewed the impact of monitoring bedside testing performed in a large medical center.2 In a 1998 review of spirometry performed outside the pulmonary function laboratory, 17% of the bedside spirometry tests met the American Thoracic Society (ATS) acceptability and reproducibility criteria. An aggressive quality improvement plan was instituted with an increase to 63.5%. The Lung Health Study, a large multicenter study using spirometry results as a key outcome parameter, also demonstrated that even well-trained technologists’ performance fell over time.3 In response to this finding, the investigators instituted a quality improvement process that included regular monitoring of test session quality and prompt technologist feedback. The authors demonstrated a dramatic improvement in test results after implementation of the quality assurance (QA) program (see Figure 1). A recent study published by Bellia and colleagues also demonstrated reproducible spirometry testing utilizing a quality feedback model in elderly subjects with and without disease,4 thus again indicating that good-quality spirometry testing can be achieved in patient populations. Quality monitoring of spirometry should begin by defining the entire path of work flow. Subsequently, a system needs to be developed that monitors the key elements along the path of work flow.
FIGURE 1. Quality assurance model. Adapted from Lung
Health Study: pulmonary function testing. Am J Respir Crit
Care Med. 1991;143:1211.
Guidance and Solutions
Three recently published documents provide solutions and recommendations for developing a quality system and expected results. The National Committee for Clinical Laboratory Standards published a proposed model for a quality system in pulmonary diagnostics.5 The path of work flow includes elements during the pretest period such as patient instructions and equipment quality control, components of the testing session, and post-test components. The document includes multiple resources and strategies for implementation of the quality system.
A spirometry position paper published by the American College of Occupational and Environmental Medicine suggests four essential components of valid spirometry. These areas include equipment performance, testing technique, measurement of results, and technician training.6
A consensus statement from the National Lung Health Education Program (NLHEP) outlines technical requirements for office spirometers.7 The statement recommends automated quality control checks (QC), messages, and grades to improve the quality of the tests. The QC thresholds are designed to be achieved by 90% of the adult patients within five maneuvers when coached by a technologist with good training, motivation, and experience. The statement also recommends the addition of educational materials to all spirometry systems used for the NLHEP.
Cleveland Clinic Improvement Plan
The Cleveland Clinic was able to make a significant impact on the quality of bedside spirometry through incorporating four key elements2:
- Providing a teaching video to all therapists performing spirometry that reviewed the ATS acceptability and reproducibility criteria.
- Limiting performance of spirometry to a “core group,” which allowed more tests to be performed per therapist.
- Providing hard-copy printouts that allow review of volume-time and flow-volume loops.
- Reviewing all tests in a central location by a pulmonary function technologist who provides constructive feedback on test quality, and reproducibility, and suggestions for improvement.
The findings and significant improvement in test quality postimplementation support the previous recommendations from the Lung Health Study.
Mayo Clinic’s Experience
Mayo Clinic has long been an advocate of quality testing in the pulmonary function laboratory (PFL) and in ancillary testing locations. Consistent policy and procedures, training, quality assurance review of testing, and technologist feedback are integral components of the Mayo Clinic’s PFL quality assurance model. Mayo Pulmonary Service (MPS), a subsidiary of Mayo Reference Services, provides quality assurance and feedback to clients utilizing spirometry testing in occupational clinics and health departments in industry throughout the country. Employing the QA model from the Lung Health Study, MPS has assisted its clients in achieving a 94% compliance with ATS acceptability and reproducibility criteria in their testing locations, once again demonstrating that quality spirometry testing can be obtained in the general population.
A 1997 review article concerning office spirometry in the Journal of Asthma emphasizes the proper training of the technologists who perform the testing as perhaps the most important factor in obtaining good spirometric testing.8 The authors also suggest that after adequate training, continued competence assessment, periodic in-services, and review of test results are important considerations. Valid spirometric results are essential in the clinical decision- making process and in the evaluation of therapeutic interventions for lung disease. Achieving validity requires a monitoring system of each of the key elements within the path of work flow.

References
1. Eaton T, Withy S, Garrett JE, Mercer J, Whitlock RML, Rea HH. Spirometry in primary care practice: the importance of quality assurance and the impact of spirometry workshops. Chest. 1999;116: 416-423.
2. Hoisington E, Stoller J, McCarthy K. Improving the quality of bedside spirometry. Respiratory Care. 1999;44:1237.
3. Enright PL, Johnson LJ, Connett JE, Voelker H, Buist AS. Spirometry in the Lung Health Study: methods and quality control. Am Rev Respir Dis. 1991;143:1215-1223.
4. Bellia V, Pistelli R, Catalano F, et al. Quality control of spirometry in the elderly: the SARA Study. Am J Respir Crit Care Med. 2000;161:1094-1100.
5. National Committee for Clinical Laboratory Standards. HS-4, proposed document. A Quality System Model for Respiratory Care. Wayne, Pa: NCCLS; 2000.
6. American College of Occupational and Environmental Medicine Position Paper. Spirometry in the occupational setting. Arlington Heights, Ill: ACOEM; 2000.
7. Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146-1161.
8. Wanger J, Irvin C. Office spirometry: equipment selection and training of staff in the private practice setting. J Asthma. 1997;34:93-104.