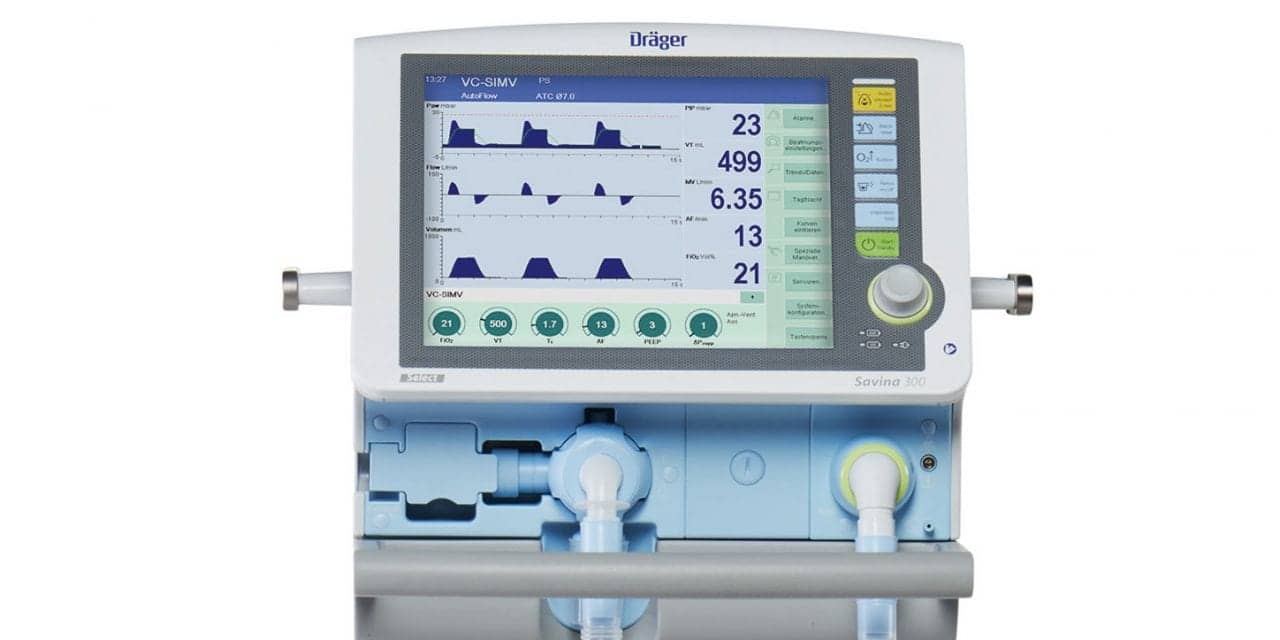
Dräger has received FDA 510(k) clearance for its Savina 300 SW 5.0, the latest in its line of Savina ventilators.
The Savina 300 SW 5.0 offers advanced features such as automatic tube compensation (ATC) to aid clinicians in their daily workflow, and improve patient comfort by reducing artificial airway resistance.
Operating independently from a central power and gas supply, the Savina 300 SW 5.0 enables a high degree of flexibility for mechanically ventilated patients in long-term facilities and acute care facilities alike.
The Savina 300 requires only ambient air, which it filters for the ventilation process. With its turbine technology, it has the ability to provide respiratory gas at a high flow delivery of up to 250 LPM. This enables the ventilator to react very quickly to pressure fluctuations and also compensate for large leakages during noninvasive ventilation.
“As long-term facilities continue to advance their clinical practices, technology must also advance to meet the needs of higher acuity patients. The Savina 300 continues to meet this growing need,” said Steve Menet, senior vice president of sales, hospital solutions, Dräger Inc.
Whether a patient is acutely ill or transitioning to long-term care, the Savina 300 covers the wide range of infant through adult patient requirements. The ventilator’s automatic tube compensation (ATC) technology compensates for resistance caused by the artificial airway to facilitate spontaneous breathing.
Patients who are critically ill can also be better served with additional sophisticated ventilation modes provided by the Savina 300, such as pressure control-airway pressure release ventilation (PC-APRV). With both integrated and external batteries, the device can operate independent of a central power supply for up to five hours.
Since their introduction in 2000, Dräger’s Savina line of ventilators have provided more than 1.4 billion ventilation hours to hospitals worldwide.