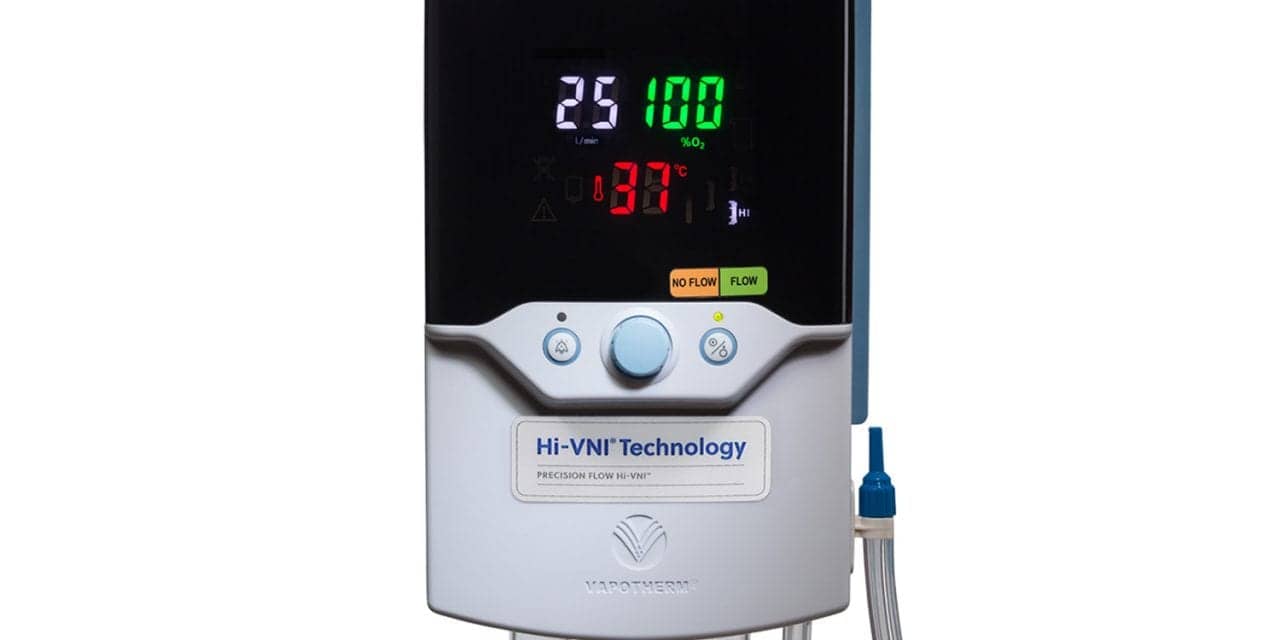
Vapotherm Inc has released the next generation of its Precision Flow Hi-VNI system.
Hi-VNI Technology is mask-free noninvasive ventilation for spontaneously breathing patients and a front-line tool for relieving respiratory distress—including hypercapnia, hypoxemia, and dyspnea. It allows for the fast, safe treatment of undifferentiated respiratory distress with one simple tool.
[maxbutton id=”1″ url=”http://info.respiratory-therapy.com/regform” text=”SUBSCRIBE TO NEWS” ]
For this generation of the Precision Flow system, both the hardware and software have been updated to improve reliability, and to comply with the EMC 4th Edition requirements for medical devices (IEC 60601-1-2:2014).
As with all the other models of the Precision Flow system, this generation provides Hi-VNI Technology, the innovation that delivers Mask-Free NIV for spontaneously breathing patients. “We’re excited to offer this next generation of the Precision Flow system to our customers,” said Joe Army, CEO. “We remain dedicated to continuous improvement in the products and services we offer our customers and patients and these improvements deliver on that commitment.”
In 2018, the US FDA granted the Precision Flow Hi-VNI system a new product category and product code (QAV). The FDA also granted an expanded indication for the system that states it may be used to augment breathing of spontaneously breathing patients suffering from respiratory distress in a hospital setting. This patient population is similar to that treated by other forms of noninvasive ventilation, making the Precision Flow Hi-VNI system a viable alternative to noninvasive positive pressure ventilation (NPPV) for most patients.