Delivery of nitric oxide via nasal cannula in nonintubated patients is safe and efficient, which helps fight the negative effects of cardiopulmonary hypertension
In the scheme of things medical, nitric oxide is a newcomer to the armamentarium. It was identified in 1987 as the substance that produces relaxation of the endothelial tissue in the body (and known as endothelial-derived relaxing factor from 1980 until 1987).1 The first fledgling experiments were done in 1989, and research activity continues today. Nitric oxide was declared the “molecule of the year”2 by Science in 1992, and one vendor’s version of inhaled nitric oxide was approved by the US Food and Drug Administration (FDA) in 1999 for use in infants (of more than 34 weeks’ gestational age) with persistent pulmonary hypertension of the newborn (PPHN).3 The FDA-approved inhaled nitric oxide is a gaseous blend of 0.8% nitric oxide and 99.2% nitrogen (which is used for stabilization of the gas).3 Inhaled nitric oxide remains an experimental therapy for all patients who do not fall into the approved category (newborns with PPHN).
Many cells in the body produce nitric oxide. Nitric oxide activates guanylate cyclase, which synthesizes a second-messenger molecule, cyclic guanosine monophosphate (cGMP). In smooth-muscle cells, the increased cGMP results in a reduction in intracellular calcium and a subsequent relaxation of the smooth muscle. Once nitric oxide enters the intravascular space, it binds to hemoglobin, forming nitrosyl iron (II) hemoglobin and then methemoglobin. Eventually, nitric oxide is converted to nitrates and nitrites in plasma and excreted, primarily by the kidney. The half-life of nitric oxide is 41 seconds,4 and since it binds to hemoglobin and becomes inactive, it has a local effect. Thus, inhaled nitric oxide is known as a potent pulmonary vasodilator.
Delivery via Nasal Cannula
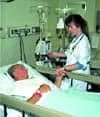
Experience with nitric oxide began at Arkansas Children’s Hospital (ACH), Little Rock, in 1993 in the cardiovascular ICU. In 1997, the Respiratory Care Services Department was given the challenge of finding a way to administer nitric oxide to patients who were not mechanically ventilated. Health care workers’ complaints of headaches and nausea when nitric oxide was delivered via hood prompted the decision to develop a low-flow nasal cannula system for use in the cardiac catheterization laboratory and the cardiovascular ICU (Figure 1).

To ensure that this was a safe way to deliver nitric oxide, ambient nitric oxide and nitrogen dioxide levels were studied.5 These ambient nitric oxide and nitrogen dioxide levels were created through the simulated administration of nitric oxide via nasal cannula. This involved running 40 ppm of nitric oxide through the delivery system in an empty patient room for 8 hours. Two environmental-air–sampling tubes were placed in the room, 61 cm and 1.52 m from the nasal prongs. The sampling tubes were replaced at hour four. The oxygen flow rate was 2.5 L/min and the nitric oxide flow rate was less than 100 mL/min. The levels of nitric oxide and nitrogen dioxide in the tubes were less than the detection limit of the laboratory (0.3 ppm). Therefore, nitric oxide levels did not exceed the US Occupational Safety and Health Administration (OSHA) permissible exposure limit of 25 ppm or the American Conference of Governmental Industrial Hygienists (ACGIH) threshold limit value of 25 ppm.5 In addition, nitrogen dioxide levels did not exceed the OSHA permissible exposure limit of 5 ppm or the ACGIH threshold limit value of 3 ppm.5
Nitric Oxide in Nonintubated Patients
Nitric oxide is used to treat nonintubated patients in the cardiovascular ICU at ACH. The original purpose of this practice was to enable cardiologists to test a patient’s pulmonary vascular response to nitric oxide during cardiac catheterization. Patients who responded to nitric oxide could be treated with it during surgery, as well as before and/or after surgery. Patients with transposition of the great vessels, an atrioventricular canal, tetralogy of Fallot, tricuspid atresia, pulmonary atresia, and total anomalous pulmonary venous return are most likely to need nitric oxide during and/or after surgery. Cardiopulmonary bypass may contribute to the pulmonary hypertension seen postoperatively in cardiac cases.6 Nitric oxide may be needed past the time of extubation in order for the patient to recover fully from pulmonary hypertension. The maximum course of nitric oxide therapy, in a study by Beghetti et al,7 was 14 days.
Questions often arise regarding oxygen delivery for these patients. At ACH, we use nitric oxide via nasal cannula with the addition of oxygen via nonrebreathing mask if the patient requires more oxygen than is delivered via the nasal cannula. Using a nonrebreathing mask will prevent any exhaled nitric oxide or nitrogen dioxide from entering the reservoir bag. If a partial-rebreathing mask were to be used, exhaled nitric oxide could enter the reservoir bag; this would allow time for the reaction with oxygen that creates nitrogen dioxide. This, plus the exhaled nitrogen dioxide already formed, could place a patient at risk for receiving a toxic dose of nitrogen dioxide.
Nitric Oxide Via Nasal Cannula
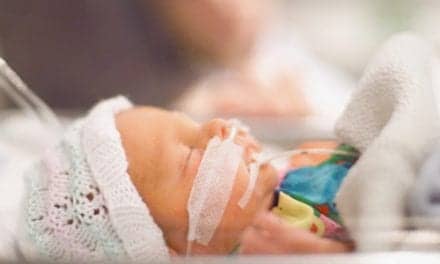
When inhaled nitric oxide was approved by the FDA in 1999, ACH replaced its older delivery configuration (Figure 2).

A delivery system for inhaled nitric oxide, cleared by the FDA in early 2000 for US distribution, draws gas through a monitoring system at a rate of 230 mL/min. Nasal-cannula flow rates set at less than 500 mL/min will not consistently deliver gas to the patient. The system, therefore, had to be adapted for use with infant/pediatric and adult cannulae. Oxygen tubing is used to connect the nipple adapter of the flowmeter to the injector module. A 4.5 mm endotracheal-tube adapter and a 22 mm male/15 mm female x 22 mm male/15 mm female adapter connect the oxygen tubing to the injector module. A step-down adapter is used to connect the outlet side of the injector module to the nasal cannula. The nasal cannula is cut approximately 15 to 25 cm ahead of the bifurcation of the cannula leading to the nasal prongs. The cut ends of the cannula are connected to endotracheal-tube adapters (2.5 mm endotracheal-tube adapters for infant/pediatric cannulae and 3.5 mm endotracheal-tube adapters for adult cannulae). One of the adapters will fit into the nitric oxide sampling adapter, while a 22 mm male/15 mm female x 22 mm male/15 mm female adapter will connect the sampling adapter to the other section of the cannula and its endotracheal-tube adapter.
This system has been used for the past 2 years without problems, but the system’s manufacturer has released an application update stating that flow rates should not be less than 1 L/min.8 The update also indicates that increasing the inhaled nitric oxide setting may cause a bolus of nitrogen dioxide to be delivered to the patient. There is no evidence of this effect occurring with the system used at ACH.
Cautions
When using nitric oxide, whether delivered via nasal cannula or ventilator, it is important to measure methemoglobin levels on a frequent basis. Normally, methemoglobin levels are obtained every 4 hours initially; after 24 to 48 hours, this will decrease to twice a day. Our protocol calls for methemoglobin levels of less than 5% (the normal range for the ACH laboratory is 0.4% to 1.5%). In clinical trials,3 methemoglobin levels were usually elevated within 8 hours of the start of nitric oxide treatment, but peaks were reached as late as 40 hours after the initiation of therapy. Methemoglobin is incapable of binding oxygen or carbon dioxide, so increases in methemoglobin will decrease the carrying capacity of the hemoglobin present in the blood. Should methemoglobin levels rise above 5%, nitric oxide should be reduced or withdrawn and methemoglobin levels should be checked frequently to determine whether the level present is decreasing following the discontinuation or reduction of nitric oxide therapy. If the methemoglobin level does not decrease, the patient may be treated with intravenous vitamin C, intravenous methylene blue, or blood transfusion, depending on the patient’s condition.3
Nitrogen dioxide is a toxic gas. Among its effects are pulmonary edema, hemorrhage, changes in surfactant, and changes in the epithelium of the terminal bronchioles.9 OSHA has set safety limits for nitrogen dioxide at 5 ppm. Nitrogen dioxide is produced spontaneously from nitric oxide and oxygen.9 There is a potential for conversion of nitric oxide and oxygen into nitrogen dioxide within lung units if the residence time is more than 7 minutes.9
Inhaled nitric oxide itself can be toxic to the lungs at high concentrations. Nitric oxide levels of less than 100 ppm are believed to be safe for most patients, with close monitoring of nitrogen dioxide and methemoglobin levels. Patients should be treated using the lowest level of nitric oxide that produces a response.
Conclusion
Delivery of nitric oxide via nasal cannula to nonintubated patients is safe and efficient. Despite the concerns that must be addressed in using nitric oxide, it is still a viable weapon in our fight to combat the negative effects of pulmonary hypertension, thereby increasing the chances of a positive outcome for our young patients.
Kathleen M. Boyle, MS, RRT, is a research therapist, Respiratory Care Services Department, Arkansas Children’s Hospital, Little Rock.
References
1. Hurford WE. The biologic basis of inhaled nitric oxide. Respir Care Clin North Am. 1997;3:357-364.
2. Koshland DE. The molecule of the year. Science. 1992;258:1861.
3. US Food and Drug Administration. INOmax (Nitric Oxide) for Inhalation. Rockville, Md: Center for Drug Evaluation and Research; 2000.
4. US Food and Drug Administration. Review and Evaluation of Preclinical Pharmacology and Toxicology Data: Nitric Oxide. Rockville, Md: Center for Drug Evaluation and Research; 1997.
5. Boyle K, Lowe G, James R. Nitric oxide (NO) via nasal cannula. Respir Care. 2000;45:1017.
6. Bettit P, Thompson J. Inhaled nitric oxide in the management of cardiopulmonary disorders in infants and children. Respir Care Clin North Am. 1997;3:459-477.
7. Beghetti M, Walid H, Friedeli B, et al. Continuous low dose inhaled nitric oxide for severe pulmonary hypertension after cardiac surgery in pediatric patients. Br Heart J. 1995;73:65-68.
8. INOvent Delivery System; INOvent Application Update #10. Madison, Wis: Datex-Ohmeda; 2000.
9. Hess D, Bigatello L, Hurford WE. Toxicity and complications of inhaled nitric oxide. Respir Care Clin North Am. 1997;3:487-498.