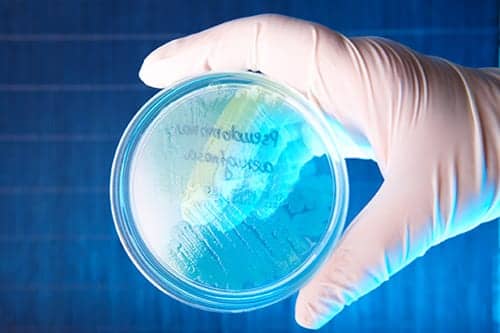
Aridis Pharmaceuticals Inc has been granted Fast Track Designation by the FDA for its fully human monoclonal antibody Aerucin for the treatment of hospital-acquired and ventilator-associated pneumonia caused by Pseudomonas aeruginosa.
Aerucin is a broadly reactive fully human immunoglobulin G, or IgG, mAb targeting P. aeruginosa bacteria that exhibits broad binding to greater than 90% of clinical isolates of P. aeruginosa. Aerucin is being developed initially as an adjunctive anti-infective to treat hospital-acquired and ventilator-associated pneumonia due to P. aeruginosa.
Aridis completed enrollment and dosing in a Phase 1 clinical study of Aerucin. Results are expected in the fourth quarter of this year.
“We are pleased to receive Fast Track designation for Aerucin as it provides an accelerated development and regulatory review pathway, and if approved, may lead to expedited availability of Aerucin to critically ill patients with hospital-acquired and ventilator-associated pneumonia,” said Vu Truong, PhD, founder and CEO of Aridis.
Fast Track designation is a process designed to facilitate the development, and expedite the review of drugs to treat serious conditions and fill unmet medical needs. Companies that receive Fast Track designation are allowed to submit New Drug Applications (NDA) or Biologics License Applications (BLA) on a rolling basis, expediting the FDA review process, and benefiting from more frequent communication with the FDA to discuss all aspects of clinical development. Additionally, drugs that receive Fast Track designation are eligible for accelerated approval and priority review.