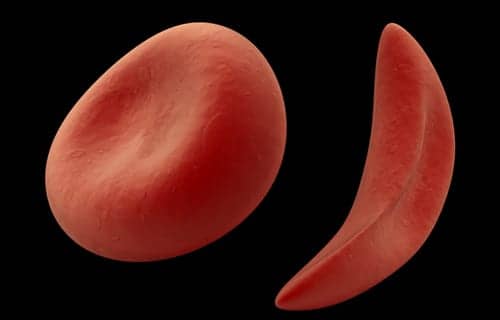
Endari (L-glutamine oral powder), a new therapy to treat sickle cell disease, is the first FDA-approved treatment for pediatric patients with the disease, and the first new SCD treatment approved by the FDA for adults in nearly 20 years, according to manufacturer Emmaus Medical Inc.
Endari will be available starting the week of December 17 through US Bioservices, a national specialty pharmacy. Endari is packaged in 5-gram packets that allow for exact dosing of 10, 20 or 30 grams daily, taken in two equally divided doses.
The drug is indicated to reduce the acute complications of sickle cell disease in adult and pediatric patients 5 years of age and older. It demonstrated a significant reduction in the number of sickle cell crises, days hospitalized, and acute chest syndrome, in a pivotal phase 3 clinical trial conducted over the 48 weeks.
“We are pleased to see that after years of research, Endari will soon be available to sickle cell disease patients in this country,” said Dr David Meyer, president and CEO of LA Biomed. “…We are optimistic about the impact this new drug will have on the lives of many patients suffering with this disease.”
More information on the drug is available at the company’s website.