A Phase 3 clinical trial has begun to determine if Moderna Inc’s investigational vaccine mRNA-1273 can prevent symptomatic coronavirus disease 2019 (COVID-19) in adults. The trial, which will be conducted at US clinical research sites, is expected to enroll approximately 30,000 adult volunteers who do not have COVID-19.
NOTE: People 18 years of age and older who are interested in participating in this trial can visit https://www.coronaviruspreventionnetwork.org or ClinicalTrials.gov and search identifier NCT04470427 for details.
“Although face coverings, physical distancing and proper isolation and quarantine of infected individuals and contacts can help us mitigate SARS-CoV-2 spread, we urgently need a safe and effective preventive vaccine to ultimately control this pandemic,” said NIAID Director Anthony S. Fauci, MD. “Results from early-stage clinical testing indicate the investigational mRNA-1273 vaccine is safe and immunogenic, supporting the initiation of a Phase 3 clinical trial. This scientifically rigorous, randomized, placebo-controlled trial is designed to determine if the vaccine can prevent COVID-19 and for how long such protection may last.”
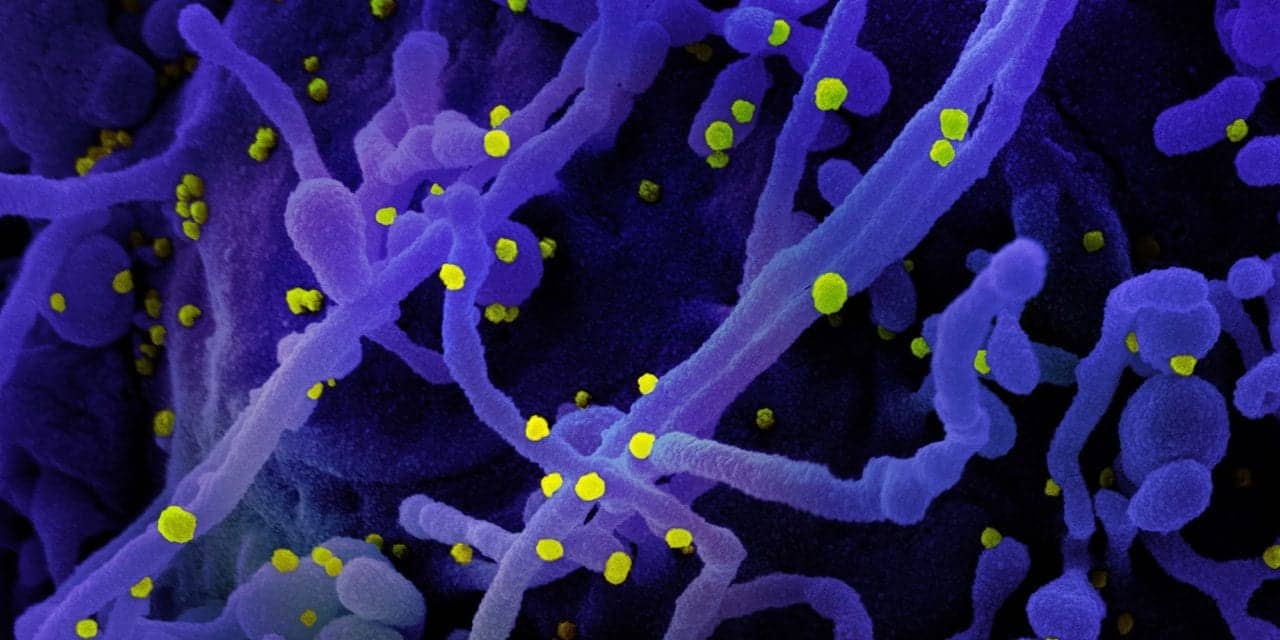
NIAID scientists developed the stabilized SARS-CoV-2 spike immunogen (S-2P). The spike protein on its surface facilitates entry into a cell. Moderna’s mRNA-1273 uses the mRNA (messenger RNA) delivery platform to encode for an S-2P immunogen. The investigational vaccine directs the body’s cells to express the spike protein to elicit a broad immune response. A Phase 1 clinical trial found the candidate vaccine to be safe, generally well-tolerated and able to induce antibodies with high levels of virus-neutralizing activity. Moderna initiated Phase 2 testing of the vaccine in May 2020.
The trial is designed to evaluate the safety of mRNA-1273 and to determine if the vaccine can prevent symptomatic COVID-19 after two doses. As secondary goals, the trial also aims to study whether the vaccine can prevent severe COVID-19 or laboratory-confirmed SARS-CoV-2 infection with or without disease symptoms. The trial also seeks to answer if the vaccine can prevent death caused by COVID-19 and whether just one dose can prevent symptomatic COVID-19, among other objectives.
Trial volunteers will receive two intramuscular injections approximately 28 days apart. Participants will be randomly assigned 1:1 to receive either two 100 microgram (mcg) injections of mRNA-1273 or two shots of a saline placebo. The trial is blinded, so the investigators and the participants will not know who is assigned to which group.
Volunteers must provide informed consent to participate in the trial. They will be asked to provide a nasopharyngeal swab and a blood sample at an initial screening visit and additional blood samples at specified time points after each vaccination and over the two years following the second vaccination. Scientists will examine blood samples in the laboratory to detect and quantify immune responses to SARS-CoV-2.
Investigators will closely monitor participant safety. They will call participants after each vaccination to discuss any symptoms and will provide participants with a diary to record symptoms and a thermometer for temperature readings.
If a participant is suspected to have COVID-19, the participant will be asked to provide a nasal swab for testing within 72 hours. If the test is positive for SARS-CoV-2 infection, the participant will be followed closely and referred for medical care if symptoms worsen. Participants will be asked to provide a daily assessment of symptoms through resolution and have saliva sampled periodically, so investigators can test for SARS-CoV-2 infection.
Study investigators will regularly review trial safety data. An independent data and safety monitoring board (DSMB) will review blinded and unblinded data—including safety data and cases of COVID-19 in both groups—at scheduled data review meetings.
Moderna is leading the trial as the regulatory sponsor and is providing the investigational vaccine for the trial. The Biomedical Advanced Research and Development Authority (BARDA), of the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response and NIAID are providing funding support for the trial. The vaccine efficacy trial is the first to be implemented under Operation Warp Speed, a multi-agency collaboration led by HHS that aims to accelerate the development, manufacturing and distribution of medical countermeasures for COVID-19.